Specific training programs for clinical research professionals often incorporate modules focused on data management, regulatory compliance, and good clinical practice (GCP). These modules might cover topics such as electronic data capture (EDC) systems, case report form (CRF) completion, and the handling of adverse events. For instance, a curriculum could include hands-on exercises simulating real-world scenarios encountered during a drug trial, such as data entry and verification procedures, ensuring data integrity and accuracy. The instruction may emphasize meticulous record-keeping and adherence to established protocols.
Effective training is crucial for maintaining the ethical conduct and scientific rigor of clinical trials. Well-trained personnel minimize errors, ensure data reliability, and ultimately contribute to the efficient and successful completion of studies. This directly impacts the validity and trustworthiness of research findings, which are vital for regulatory submissions and the advancement of medical knowledge. Historically, the increasing complexity of clinical trials, coupled with stricter regulatory oversight, has necessitated more comprehensive and specialized professional development opportunities.
Subsequent sections will delve into specific aspects of clinical trial training, examining best practices in data management, regulatory compliance, and the ethical considerations involved in conducting research involving human subjects. The importance of continuous professional development in this rapidly evolving field will also be addressed.
Images References
Source: www.slideteam.net
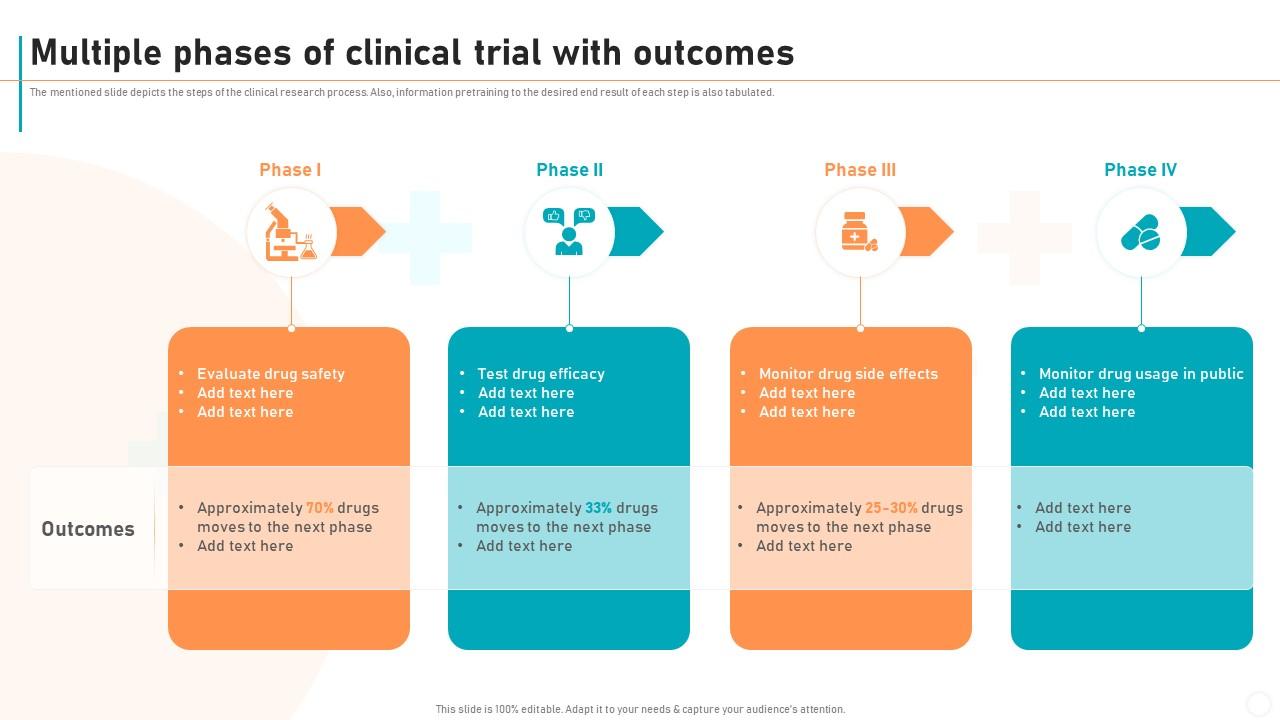
Multiple Phases Of Clinical Trial With New Drug Development

Source: ccrps.org
Clinical Research Education and Training Requirements Clinical
Leave a Reply