Both USP and ISO standards represent distinct approaches to quality assurance, each with its own rigorous requirements. USP (United States Pharmacopeia) standards focus primarily on pharmaceutical products and ingredients, emphasizing purity, potency, and safety for human and animal use within the United States. ISO (International Organization for Standardization) standards, on the other hand, provide a broader framework encompassing various industries and products, with a focus on quality management systems and consistent operational excellence. A direct comparison of stringency is difficult, as they address different aspects of quality and compliance, operating under different scopes and jurisdictions.
The importance of these certifications lies in demonstrating a commitment to consistent quality and adherence to internationally recognized best practices. For pharmaceutical companies, USP compliance is often a regulatory requirement, ensuring patient safety and product efficacy. ISO certifications, while not always mandatory, offer significant competitive advantages, enhancing credibility with customers and demonstrating a proactive approach to operational excellence across the organization. Historically, the evolution of these standards reflects a growing global emphasis on quality control and consumer protection.
Subsequent sections will delve into a detailed comparison of the specific requirements, application processes, and overall impact of each certification, enabling a more informed understanding of their respective roles in ensuring product quality and safety.
Images References
Source: www.abptech.com
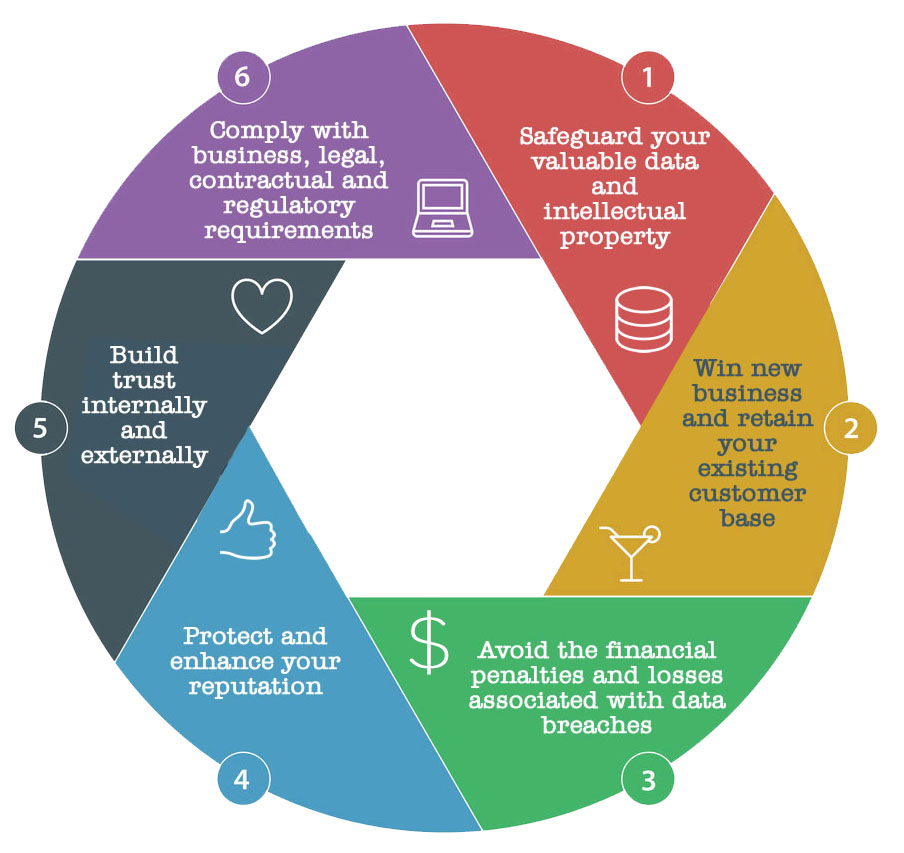
Look for ISO 27001 Certification The Gold Standard in Data Processing
Source: techgeekers.com
What Is ISO 27001 Consultancy And Its Importance TechGeekers
Leave a Reply