Solvent selection is critical in numerous chemical processes, particularly in those involving the separation, purification, or reaction of compounds. The choice of solvent depends heavily on the chemical properties of the target compound, such as its polarity, hydrogen bonding capability, and molecular weight. For instance, a polar compound like a salt will readily dissolve in a polar solvent such as water, while a nonpolar compound like a lipid will be more soluble in a nonpolar solvent like hexane. Understanding these interactions is fundamental to efficient and effective chemical manipulation.
Appropriate solvent selection optimizes reaction yields, facilitates efficient purification techniques like recrystallization or extraction, and minimizes waste generation. Historically, the understanding of solubility principles has driven innovation in various fields including pharmaceuticals, materials science, and environmental remediation. The ability to dissolve a target molecule efficiently and selectively remains a cornerstone of many scientific and industrial endeavors, impacting the development of new drugs, the creation of advanced materials, and the mitigation of environmental pollutants.
The following sections will delve into the principles governing solubility, explore various solvent properties, and provide practical guidance for determining the optimal solvent for a given compound based on its structural characteristics and desired application. Specific examples will illustrate the selection process, highlighting the interplay between molecular structure and solvent choice.
Images References
Source: www.chegg.com
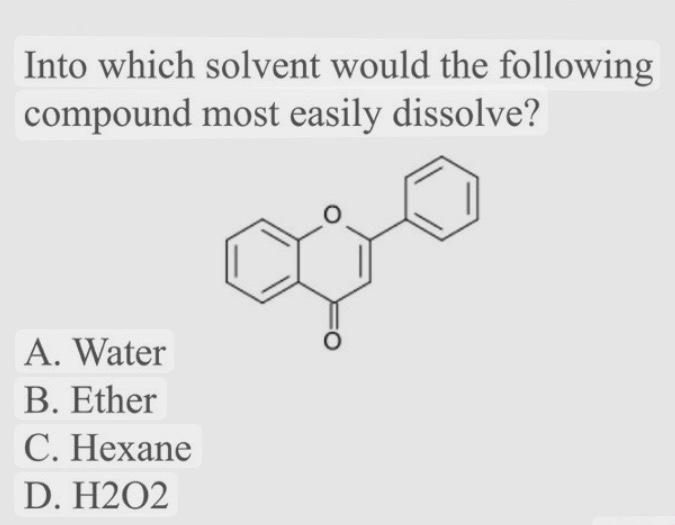
Solved Into which solvent would the following compound most
Source: www.chegg.com
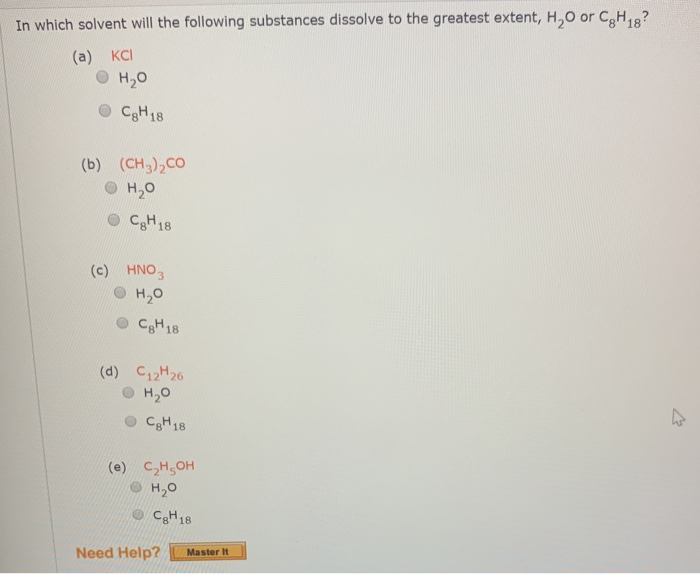
Solved In which solvent will the following substance
Leave a Reply